“V-Day” Arrives In UK As First ‘High-Risk’ Patient Vaccinated; US COVID Hospitalizations Climb: Live Updates
Tyler Durden
Tue, 12/08/2020 – 08:21
Summary:
- UK vaccinates first patient: a 90-year-old grandmother
- FDA releases data claiming Pfizer vaccine merits approval
- US hospitalizations, average new cases, hit new highs
- CT now home to most new cases/per capita
- India says vaccine licenses coming
- Hong Kong to ban indoor dining after 1800
- Tokyo reports 352 new infections
- Brazil in talks to buy 70 million vaccines
- Pfizer, Moderna warn about inability to deliver more doses
The big day has finally arrived.
Less than a week after becoming the first western country to approve a COVID-19 vaccine for emergency use, the UK has confirmed the vaccination of the first patient to receive the vaccine outside of a clinical trial setting in the west. Kicking off a day that HMG has branded “V Day”, a historical allusion to the allied triumph in World War II. To try and combat burgeoning doubts about the safety and efficacy of the first round of COVID vaccines (which rely on a relatively new mRNA technology), authorities selected a 90-year-old woman, who became the first patient to receive the vaccine.
The patient’s name is Margaret Keenan, and she’s a 90-year-old grandmother.
“If I can do it, so can you”: this 90-year-old UK grandmother has become the first person in the world to receive the Pfizer-BioNTech vaccine outside of trials https://t.co/E11VI2TNXE pic.twitter.com/3hzNL2gzzh
— SCMP News (@SCMPNews) December 8, 2020
As the Associated Press declared in its report on the vaccination, Tuesday is “a momentous day in the global fight against the coronavirus pandemic that has killed more than 1.5MM people and infected over 67 million more in 2020, the worst health crisis the world has faced in a century.”
British officials have already distributed nearly a million doses of the Pfizer vaccine across the UK, and injections are beginning in all four of the UK’s constituent nations.
The first shot was administered at one of the hospital hubs around the country on what UK officials dubbed “V-Day,” Danica Kirka reports from London.
Meanwhile, in the US, the FDA published a report Tuesday morning from the panel convened yesterday to start examining the data from the Pfizer-BioNTech vaccine trials. They happily declared that there’s “no reason” to delay emergency-approval of the vaccine. The agency is expected to release two reports analyzing the Pfizer-BioNTech vaccine ahead of Thursday’s meeting, with data expected to break down the vaccine’s efficacy with various age, ethnic and other demographic groups.
On Thursday, the FDA’s vaccine advisory panel will discuss these materials in advance of a vote on whether to recommend authorization.
Finally, a report from WaPo published last night claimed that Pfizer and Moderna won’t be able to supply the US with additional doses until the late spring/summer. While the implication was that this was some kind of retribution from the Trump Administration, former FDA Dr. Scott Gottlieb explained on CNBC Tuesday morning that this was likely not the case.
“How good are the vaccines at preventing infection and preventing transmission? We know they prevent #COVID19 disease … but how good are they at actually preventing people from getting infected in the first place? @ScottGottliebMD on what questions we still need to answer. pic.twitter.com/MP0rK8Qo0Q
— Squawk Box (@SquawkCNBC) December 8, 2020
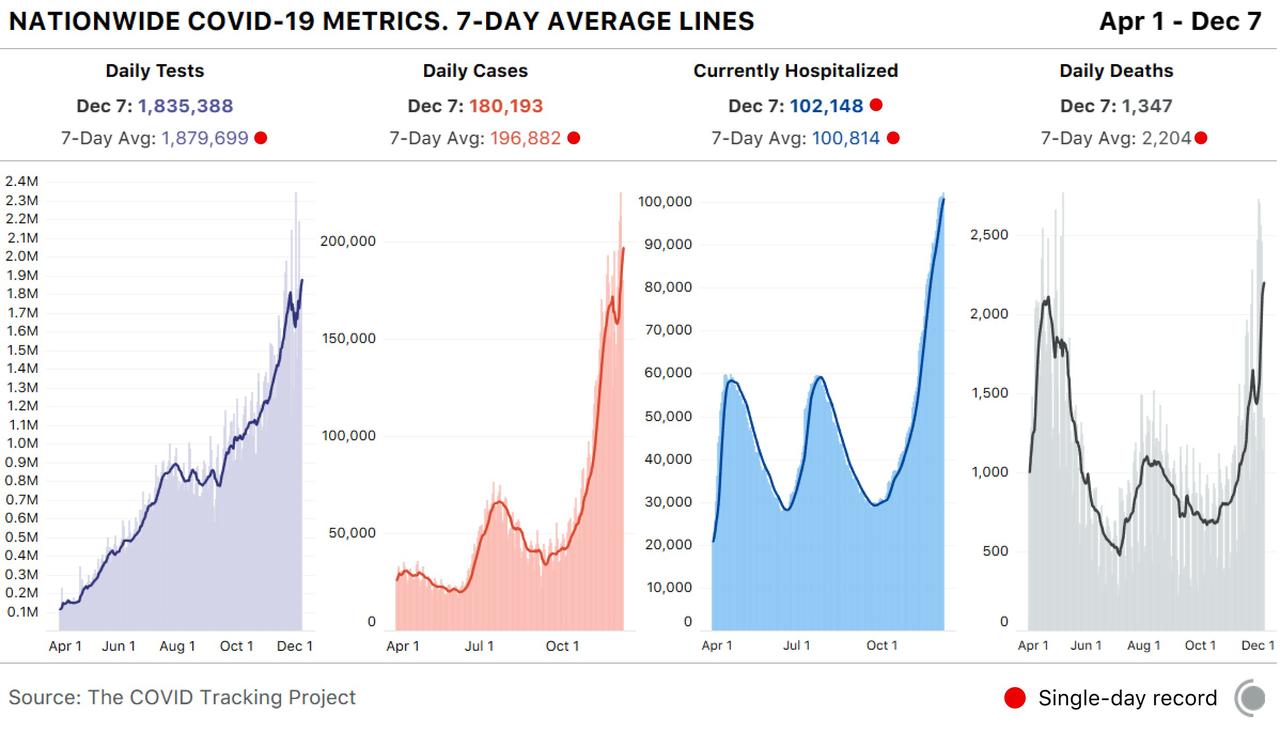
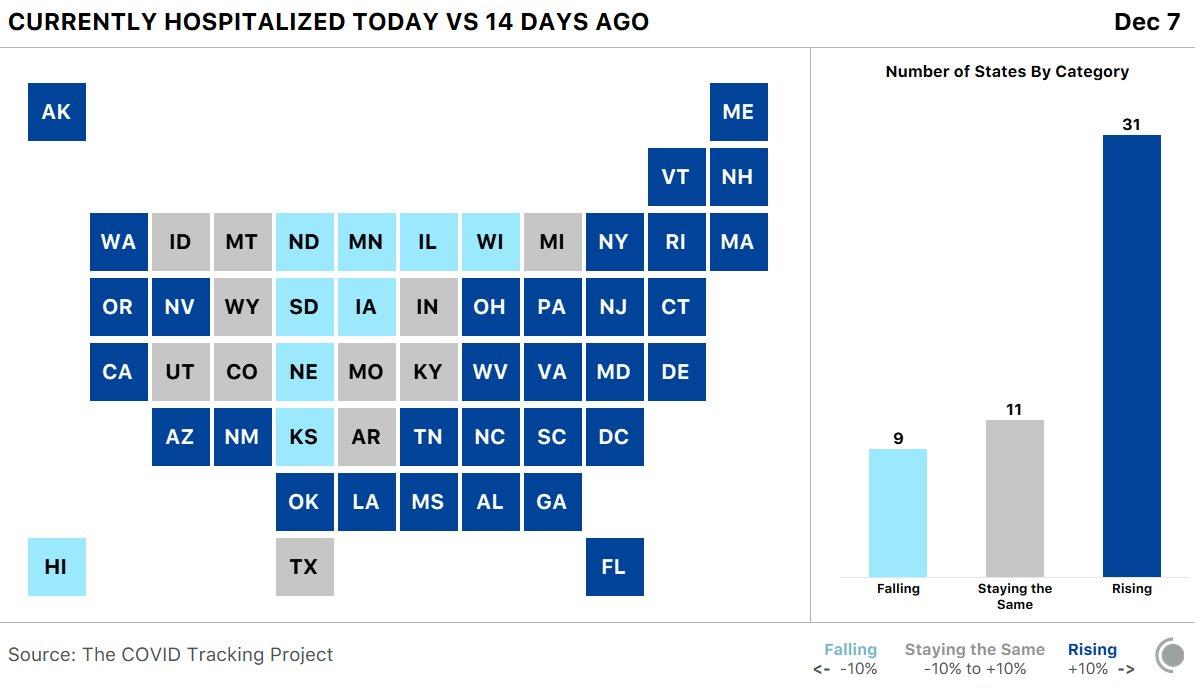
Circling back to the state of the US outbreak, hospitalizations nationwide climbed to a fresh record high…
…Even as some of the most worrisome midwestern and mountain west states – including WI, IL, ND & SD – have seen cases and hospitalizations slow recently.
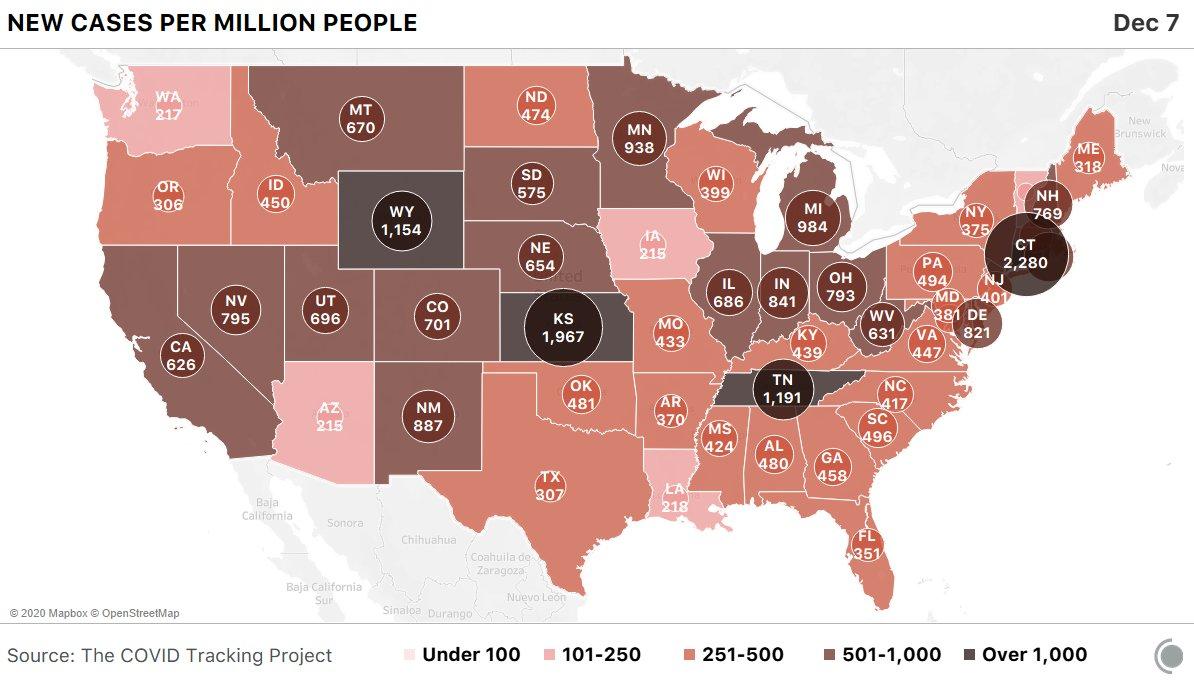
Amazingly, tiny Connecticut, a state that was widely praised for it initial response to the virus, is now seeing the highest case count per capita as testing ramps up.
Across the US, states are continuing to tighten restrictions as more than 30MM people are now under lockdown in and around LA as new ‘stay at home orders’ are handed down in the Golden State.
Here’s some more COVID news from overnight and Tuesday morning:
India’s federal health secretary Rajesh Bhushan says the government’s regulator could grant a license to some developers of COVID-19 vaccines in the next few weeks. Six vaccines, including Astra Zeneca’s Covidshield and Bharat Biotech’s Covaxin, are in trial stages, Bhushan says (Source: Nikkei).
Indonesia’s state-owned pharmaceutical company Bio Farma says that interim data on trials it was conducting on vaccines produced by the Chinese company Sinovac showed up to 97% efficacy (Source: Nikkei).
Tokyo reports 352 new infections, up from 299 a day earlier, with the number of patients in serious condition in the capital increasing by five to 60 (Source: Nikkei).
India reports 26,567 cases in the last 24 hours, the lowest daily count since July 10, bringing the country’s total to 9.7 million. The death toll jumped by 385 to 140,958 (Source: Nikkei).
Hong Kong will ban dining in restaurants after 6 p.m. to curb a rise in coronavirus cases in the densely packed financial hub (Source: Nikkei).
Brazil’s government says it is in advanced talks with Pfizer to buy 70 million doses of COVID-19 vaccine, and a memorandum of intent should be signed this week (Source: Brazil).
* * *
Britain of course isn’t the first country to start vaccinating under emergency order: China has already vaccinated well over 1 million people, and Russia on Saturday started vaccinating health-care workers and other ‘vulnerable’ individuals in Moscow, the epicenter of what has become one of the largest outbreaks in the world.
via ZeroHedge News https://ift.tt/3lXeyP0 Tyler Durden