“It Really Is THAT Good” – Wall Street Reacts To Merck’s “Big Deal” Drug
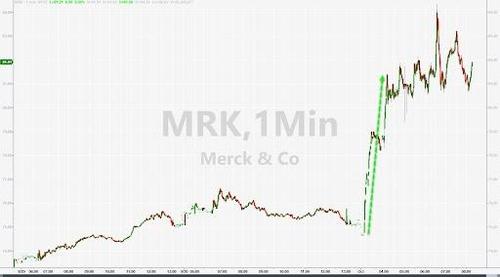
As we reported earlier this morning, Merck has released what experts say are “extremely positive” test results for a COVID anti-viral that cuts risk of hospitalization in half, while also dramatically reducing the risk of death. The data were so profoundly positive that an oversight board ended the trial, claiming that withholding the drug from patients in the placebo group would be “unethical”.
Merck says it can deliver 10MM doses of the new drug, called Molnupiravir, by the end of the year, by which it should be approved by the FDA and possibly foreign regulators as well (the wheels of bureaucracy are reportedly turning as quickly as they can). Merck is submitting an emergency application for authorization of the drug, and we may see it in use during the next two weeks. The regiment is 2 pills a day for 5 days, and it’s most helpful within 5 days of infection.
The news has sent US stocks into the green for the day, while shares of Merck briefly soared as much as 12%.
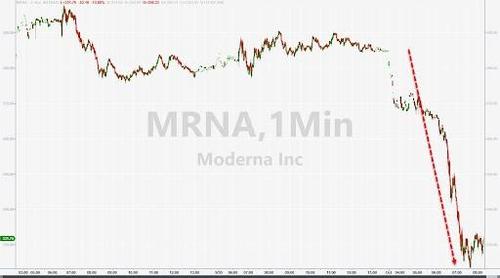
Meanwhile, shares of Moderna are really taking it on the chin, and have fallen by more than 10%.
After failing to foresee the potential “game-changing” impact of the new drug, Wall Street analysts are scrambling to interpret what Molnupiravir will mean for markets and the economy.
In a roundup of commentary from Bloomberg, most agreed that the drug would be a “game-changer” for the pandemic, and that it will likely receive emergency use authorization before the end of the year.
Evercore ISI analyst Umer Raffat referred to the pill as a game changer for Covid-19, writing that the data “really is THAT good”.
- Expects oral pills to be a “big deal” in combating Covid with the ease of use and scale of manufacturing being a game changer
- From MRK stock perspective, pipeline should finally start to get real credit – starting with this readout today”
Barclays analyst Carter Gould expects a straightforward regulatory decision for the pill to receive emergency use authorization from the FDA with the data representing a needed win for Merck in combating Covid-19.
- Highlights that the Street had estimated near-zero value for the drug prior to today’s news with Merck set up well to book a $1.2 billion contract later this year.
Cantor analyst Louise Chen highlighted that the data showed consistent efficacy across Gamma, Delta, and Mu variants and said the pill could “change the treatment paradigm”
- “Positive data could clear path to take a slice of an untapped pie in the COVID treatment landscape (multi-blockbuster opportunity in the U.S. alone)”
SVB Leerink analyst Daina Graybosch wrote that the drug could unlock more than $10b in near-term orders and also “represent the best option for bringing the pandemic under control worldwide”.
- Expects approval in the U.S. before year-end with additional supply contracts in the coming weeks
Morgan Stanley’s Matthew Harrison wrote that the data are a significant positive for patients and the broader public’s risk perception as it relates to COVID
* * *
Source: Bloomberg
Tyler Durden
Fri, 10/01/2021 – 12:40
via ZeroHedge News https://ift.tt/3FkjIj5 Tyler Durden