Pharmaceutical Companies Expanding mRNA Technology To Treat Influenza
Authored by Meiling Lee via The Epoch Times (emphasis ours),
Unlike other vaccines, influenza shots are administered yearly, yet offer generally lower protection than most routinely recommended vaccines, from as low as 10 percent to as high as 60 percent. This season’s flu jab was only 16 percent effective, according to the Centers for Disease Control and Prevention (CDC).
Scientists had hoped to develop a universal flu vaccine that could fight against many strains of the flu and be given every five or 10 years, eliminating the need for annual shots. They said it could take eight to 10 years for the universal flu vaccine to be available, but over a decade later, the research did not bear fruit.
Now, major pharmaceuticals are hoping to change the shot’s underwhelming protection and the current flu manufacturing process with the messenger RNA (mRNA) technology platform.
If the companies are able to succeed in this, the “new flu jabs could prove lucrative or help maintain standing in a global market projected to exceed US$10 billion by decade’s end,” according to an article published in Nature.
Dr. Robert Malone, who helped invent the mRNA vaccine technology, said that flu shots are a “significant commercial market in the United States” that is annually recommended to “drive a market need so that the manufacturing plants can be kept running and certified” in the possibility that an outbreak similar to the 1918 influenza pandemic should occur.
“The government wants to be able to ensure that they always have enough influenza capacity in case the bad thing happens,” Malone told The Epoch Times. “So the way they do that is they create market incentives for manufacturers to produce seasonal influenza vaccines, even though we don’t really need them.”
Fortune Business Insights, offering market studies and consultation services, reported that the flu vaccines generated about $5.86 billion in 2020 and $6.59 billion globally in 2021, and is projected to grow to $10.73 billion in 2028 at a CAGR [compound annual growth rate] of 7.2 percent.
“The coronavirus pandemic naturally impacted the routine immunization programs and campaigns conducted worldwide in developing and developed countries,” the company said in its report. “However, Flu vaccination rates have gone up considerably during the pandemic owing to factors such as push from health experts/health departments as well as extension/expansion of various government programs that provide free vaccination against the flu.”
Seqirus, one of the largest manufacturers of flu shots in the world and owned by CSL Limited, experienced a sales growth of its vaccines despite the pandemic, with the company’s chief executive officer reporting (pdf) total revenue of “over $1.7 billion, up 30% at constant currency” for the fiscal year 2021 that was “driven by the very strong sales growth in seasonal influenza vaccines of some 41%.”
Differentiating itself from its competitors, Seqirus is developing flu vaccine candidates based on the next generation of mRNA technology, self-amplifying messenger RNA (sa-mRNA).
Like mRNA vaccines that instruct the cells in the body to make a protein that stimulates the immune response to build up immunity, sa-mRNA also “instructs the body to replicate mRNA, amplifying the amount of protein made.”
“This could enable vaccine manufacturers to potentially develop more effective vaccines with a smaller dosage and with lower rates of reactogenicity, underscoring the application in both pandemic and seasonal settings,” the vaccine maker announced in a press release in August 2021.
Seqirus said it would begin clinical trials of its seasonal and pandemic flu vaccine candidates in the second half of 2022.
Pfizer, Moderna, and Sanofi began testing their mRNA flu vaccines in adults 18 and older in 2021, while Curevac announced last month it was conducting a small Phase 1 trial in Panama.
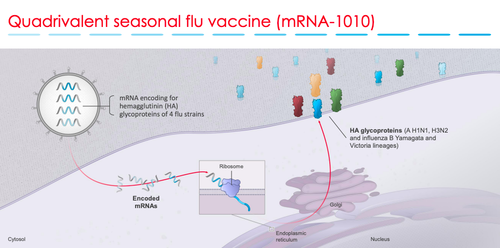
Moderna released interim data for the Phase 1 trial of its quadrivalent (four-strain) seasonal flu vaccine candidate, mRNA-1010 in December 2021, and announced that its two-dose vaccine produced robust antibody titers with “no significant safety concerns” observed, even at the lowest dose of 50 micrograms.
While Moderna’s vaccine candidate may have shown it produced antibodies after two doses, it was not as robust in the older patients who were given the available one-dose traditional flu vaccine from Sanofi, Fluzone. After a presentation of its data to investors, Moderna’s shares tumbled 10 percent after a sharp selloff of its shares that same day, according to Reuters.
In addition to the inferior antibody productions, all three various doses of Moderna’s vaccine elicited more adverse events compared to placebo in both the 18 to 49 age group and those 50 and older.
In the 100 microgram dose (which is the same dose used in its COVID-19 vaccine), Malone said that “90 percent of the people in this study developed adverse events compared to 30 percent in the control group. Of those adverse events, a large fraction of them was grade 3 out of 4, grade 4 being deaths or hospitalizations.”
He added, “So what we learned was that the toxicity associated with the mRNA tech that’s being deployed globally right now, it’s not just due to the spike protein … but it’s also due to the underlying components.”
A former Sanofi executive, Gary Nabel, told Nature that mRNA may be a “tool that offers some upside potential” but the “big stumbling block is safety.”
The lipid nanoparticles (LNPs) that encapsulate mRNA so that it can successfully enter cells have been shown to be highly inflammatory in a study by researchers from Thomas Jefferson University and published on Cell Press (pdf).
“Overall, the robust inflammatory milieu induced by LNPs, combined with presentation of the vaccine-derived peptides/protein outside of antigen-presenting cells, might cause tissue damage and exacerbate side effects,” the authors wrote. “Because self-antigen presentation in an inflammatory environment has been linked to autoimmune disease development, this merits further investigation.”
Effectiveness
The Centers for Disease Control and Prevention (CDC) reported that this season’s flu vaccine offered very little protection against mild to moderate influenza illness.
In a study of 3,636 children and adults in seven states from October 2021 to February 2022, the CDC said that the vaccine was only 16 percent effective, a rate that the health agency considers “not statistically significant.”
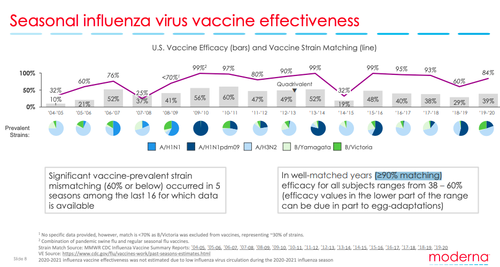
The effectiveness of the flu shot has typically been around 40 percent to 60 percent since the CDC first began estimating and tracking it in 2004.
Breaking it further down, however, we see that of the 18 seasons that the CDC has been tracking vaccine efficacy, only one season (2010–11) was 60 percent. Whereas in eight seasons, it was around 40 percent to 56 percent, and seven seasons saw lower than 40 percent effectiveness, with the lowest being 10 percent in 2004–05.
The CDC did not publish a report for the 2008–09 season but claimed that the vaccine effectiveness was 41 percent, and for 2020–21, the federal agency said it did not estimate the effectiveness of the shot because there was “low flu virus circulation” during that season.
This season’s flu shot has been updated to include four different virus components that are either inactivated or live-attenuated. Two of the components consist of subtypes of the influenza A virus, and the other two are lineages of the influenza B virus.
Experts say that this season’s low vaccine efficacy stems from a mismatch between the vaccine virus components and the circulating viruses. Yet Moderna shows that even in “well-matched years (≥90% matching), efficacy for all subjects ranges from 38-60%” noting that lower efficacy values less than 40 percent may be due in part to egg-adaptations.
Despite this, the CDC says that as long as the flu season is not over, people 6 months and older should still get vaccinated, except when contraindicated, because it may “prevent serious outcomes.”
Flu season usually runs from October to May and peaks between December to March in the United States. There has been a slight uptick in flu activity across the country with at least 2.7 million illnesses, 26,000 hospitalizations, and 1,500 deaths reported thus far for this season but still below baseline, according to the CDC.
Evolution of Flu Vaccines
The flu vaccine was initially developed for American soldiers in the 1940s and upon its approval in 1945, it became available for the general public a year later. But it wasn’t until 1964 when the vaccine was specifically recommended by federal health authorities to individuals at high risk for flu complications. Flu shots were later expanded to include people in contact with high-risk patients in 1986.
By 2010, the flu vaccine was recommended for every healthy American 6 months and older, a move that was claimed to be based on “expert and organizational opinion” rather than on solid clinical data, according to an in-depth 2012 report (pdf) by scientists at the Center for Infectious Disease Research and Policy at the University of Minnesota.
“The movement toward a universal recommendation for vaccination did not occur primarily as a result of a preponderance of newly published evidence; rather, changes were made in part on the basis of expert and organizational opinion,” the authors wrote.
“Furthermore, the ACIP [Advisory Committee on Immunization Practices] have not always accurately reflected the evidence used to support the recommendations and routinely have cited studies with suboptimal methodology (eg. that use serology as an endpoint for infection among [trivalent inactivated vaccine] recipients) as supportive,” they added.
ACIP, established in 1964, is a committee within the CDC, consisting of experts from the medical and public health fields, that makes recommendations on how to use vaccines.
The 2012 report was not the first study to comment on the shortcomings of the flu vaccine. A 2010 Cochrane review analyzing data that looked at the effects of administering healthy adults with the flu vaccine, found that “vaccination had a modest effect on time off work and had no effect on hospital admissions or complication rates.” In addition, it also found that “inactivated vaccines caused local harm and an estimated 1.6 additional cases of Guillain‐Barré Syndrome per million vaccinations. The harms evidence base is limited.”
In an updated 2018 review, the Cochrane authors examined 52 studies of over 80,000 participants but only focused on data from 25 clinical trials after they were not able to “determine the impact of bias on about 70% of the included studies due to insufficient reporting of details.”
The authors found that the flu vaccine probably had a “small protective effect against influenza and ILI [influenza-like illness] (moderate-certainty evidence), as 71 people would need to be vaccinated to avoid one influenza case, and 29 would need to be vaccinated to avoid one case of ILI.”
They also added, “Vaccination may have little or no appreciable effect on hospitalizations (low-certainty evidence) or number of working days lost.”
Tyler Durden
Wed, 03/23/2022 – 19:00
via ZeroHedge News https://ift.tt/b39FCPe Tyler Durden