Pfizer Tumbles After Abandoning Weight-Loss Pill
With shares already reflecting a post-COVID dearth of revenue streams, Pfizer’s growth outlook just took another hit as it halted development of its experimental weight-loss pill after a high rate of side effects appeared in a mid-stage study.
More than half of patients in the study had to stop taking danuglipron due to nausea and vomiting, according to a statement Friday.
While the most common adverse events in the study were mild, nausea was seen in up to 73% of patients, vomiting in up to 47% and diarrhea in as much as 25%.
Pfizer will continue developing a once-daily version of the pill in hopes that it may improve its tolerability profile.
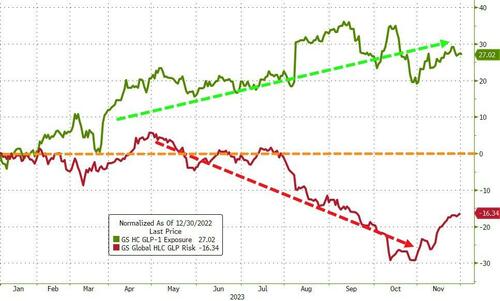
Pfizer had hoped to follow Eli Lilly and Novo Nordisk’s lead (both of which provide the shot-based GLP-1 agonists that have made so much news this year) and driven massive outperformance…
Pfizer and AstraZeneca saw pills as a way to make inroads into a market projected to reach $100 billion within seven years. Maybe not so much now…
Pfizer CEO Albert Bourla has said that oral weight-loss drugs will capture a third of the obesity market.
The company had pinned its weight-loss hopes on danuglipron after discontinuing another experimental obesity drug in June due to safety concerns.
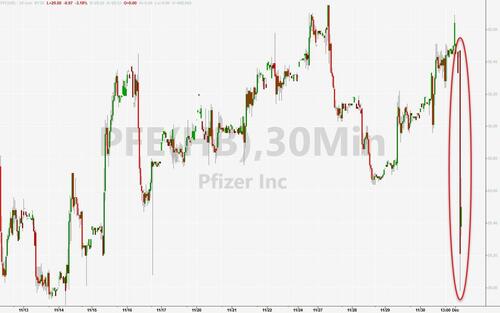
Pfizer shares are down around 4% in the pre-market, back near their lowest since March 2020 on the back of this news…
Presumably, they did not have a liability waiver this time…
Tyler Durden
Fri, 12/01/2023 – 07:20
via ZeroHedge News https://ift.tt/uVbjFnL Tyler Durden