Novo Nordisk Crashes Most On Record After CagriSema GLP-1 Results Disappoint
Novo Nordisk’s top-line efficacy results for its experimental obesity drug, CagriSema, were just released, missing the pharma’s estimate of 25% weight loss over 68 weeks, coming in at just 22.7%. This sent shares of Novo in Europe crashing the most on record.
The results:
When evaluating the effects of treatment if all people adhered to treatment1, people treated with CagriSema achieved a superior weight loss of 22.7% after 68 weeks compared to a reduction of 11.8% with cagrilintide 2.4 mg, 16.1% with semaglutide 2.4 mg and 2.3% with placebo alone. In addition, 40.4% of patients who received CagriSema reached a weight loss of 25% or more after 68 weeks, compared to 6.0% with cagrilintide 2.4 mg, 16.2% with semaglutide 2.4 mg, and 0.9% with placebo.
Novo previously forecasted the experimental obesity drug would help patients achieve at least 25% weight loss.
Meanwhile, Goldman’s James Quigley was most bullish on the street, with an estimated 27-28% weight loss.
To start the week, we penned a note telling readers about Novo’s “final big market cap event of the year” ahead of top-line efficacy results …
“Final Big Market Cap Event Of Year”: All Eyes On Novo’s CagriSema GLP-1 Results https://t.co/ALZzX5pXoN
— zerohedge (@zerohedge) December 17, 2024
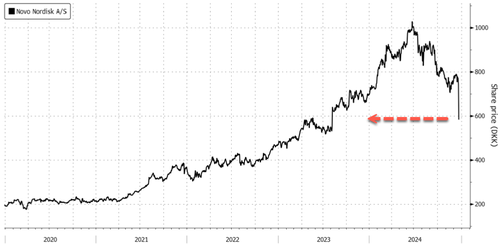
The dismal results led Novo shares to crash as much as 29% in Europe—the most on record.
This wiped out around $120 billion in market capitalization.
Results also sparked volatility with other weight-loss drug developers in Europe and the US (courtesy of Bloomberg):
-
Shares of smaller weight-loss drug developers also drop in Europe: Zealand Pharma -21%, Gubra -23%
-
Medical packaging companies also fall: Gerresheimer -14%, Bachem -6.5%
-
In US premarket trading, shares of obesity-drug rivals gain: Eli Lilly +12%, Viking Therapeutics +12%
Novo bulls received their Christmas gift early: coal.
Tyler Durden
Fri, 12/20/2024 – 06:42
via ZeroHedge News https://ift.tt/1Foeaqp Tyler Durden