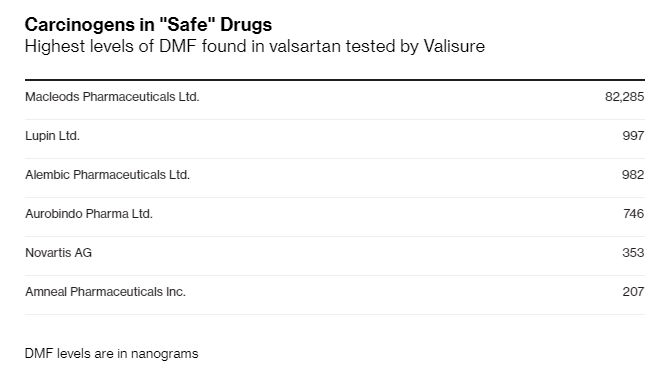
A fourth cancer-causing compound was found in widely prescribed blood pressure pills, according to a new report by Bloomberg. The solvent dimethylformamide was discovered in the drug valsartan, manufactured by several companies including Novartis, by an online pharmacy, Valisure.
The solvent, also known as DMF, is classified by the World Health Organization as a probable carcinogen.
This adds to the worry about valsartan, which has seen dozens of its generics recalled since July 2018, when the carcinogenic chemical N-Nitrosodimethylamine (NDMA) was detected in a version of the drug made by a Chinese company. Valsartan is a treatment for hypertension that is frequently combined with other medicines into a single pill, and has been around for decades.
Online pharmacy Valisure found DMF in the drug that is still on the market in the US, including in medicines that the FDA had highlighted as alternatives to recalled drugs. These findings obviously complicate the FDA’s efforts to remove the drug from pharmacies while keeping doctors and patients abreast as to what is safe.
Novartis spokesman Eric Althoff said: “Novartis cannot currently fully exclude the possibility that traces of DMF (within acceptable limits) may have been present in materials.”
The FDA is currently investigating how the recalled medications were contaminated, including the possibility that the use of DMF in the manufacturing process may have led to chemical reactions that formed the other carcinogens.
The FDA is going to evaluate Valisure’s study, but is telling patients that they should continue taking the blood pressure medication even if it’s recalled, until they can talk to their doctor. The findings of the online pharmacy suggested the manufacturing of generic drugs may involve more parties than commonly thought.
Valisure Chief Executive Officer David Light said: “Medicines are kind of like used cars: By the time you get it it’s already five or six years old, it’s touched hundreds of hands and it’s got 100,000 miles on it.”
When drugs are made, the raw materials may need to change form before they go into a pill. Often times, a solvent like DMF can be used, but it is supposed to vanish by the time a pill is put into a bottle for sale. DMF is cheaper than some other solvents, which obviously gives it appeal for generic drug making, which is already a capital intensive business.
Valisure found DMF in valsartan made by five of the six drugmakers it tested. This includes in Novartis’ Diovan, which was first approved in the US in 1996. As Bloomberg noted, the FDA doesn’t regularly test pharmaceuticals, relying instead on companies to ensure that medicines work and are safe.
Suma Thomas, a cardiologist at the Cleveland Clinic, said: “I think we make assumptions that drugs, once they’re approved by the FDA or once they’re on the market for patients, that they’re OK and they’re all the same. We need more knowledge.”
via ZeroHedge News http://bit.ly/2WTuuKy Tyler Durden