Supplies Still Limited More Than A Year After Monoclonal Antibodies Authorized For Treating COVID-19
Authored by Meiling Lee via The Epoch Times (emphasis ours),
Despite being the only authorized outpatient medical therapy for preventing the worsening of COVID-19 symptoms in high-risk patients, there remains no steady supply of monoclonal antibodies from the federal government a year after its approval for use by medical regulators.
Rolled out in the same month as the COVID-19 vaccines, monoclonal antibody therapies have not gotten the attention that vaccine treatments have after they were billed as the thing to get America out of the pandemic.
Even today, President Joe Biden continues to mostly focus on vaccinating children, providing boosters to every adult, and increase testing as part of his “new actions” to combat the COVID-19 pandemic during the winter.
Dr. Marc Siegel, a practicing internist and a professor of medicine at NYU’s Langone Medical Center, says vaccines alone cannot bring America out of the pandemic. More breakthrough cases are occurring with the Delta and Omicron variants, and hospitalizations are rising this winter.
More than 206 million Americans are fully vaccinated and 72 million people have received a booster dose as of Jan. 5. Individuals are considered fully vaccinated if they received two doses of the messenger RNA vaccines or a single shot of the Johnson & Johnson vaccine.
Siegel said that the Biden administration has not been able to deliver any therapeutics for early treatment of people with mild or moderate COVID-19 because of the administration’s “obsessive focus on the vaccines to the exclusion of all else.”
“When it comes to therapeutics, the Biden team’s results are even more anemic,” Siegel wrote in an op-ed in USA Today. “Paxlovid, Pfizer’s new protease inhibitor wonder drug, has been approved but is scarce.”
“Ditto monoclonal antibodies, the synthetic neutralizing antibodies that have been so helpful in patients at high risk of complications or hospitalizations. Omicron is most susceptible to sotrovimab, made by GlaxoSmithKline, but in most states, it is almost impossible to find,” he added.
The Epoch Times has reached out to the Biden administration for comment.
The U.S. Department of Health and Human Services (HHS) has delivered over 197,000 courses of the monoclonal antibody treatments to state and territorial health departments, and to federal entities for the week of Jan. 3. This comes after the agency briefly paused distribution of the treatments on Dec. 23.
Lack of Public Messaging
The monoclonal antibody therapies have been shown to be effective in preventing severe disease or hospitalization in high-risk patients with mild to moderate COVID-19. They are different from the convalescent plasma from a recovered COVID-19 patient, in that monoclonal antibodies are “created [in a lab] to specifically target an essential part of the infectious process,” according to physicians from the University of Michigan Health System.
As of Dec. 16, 2021, the National Institutes of Health does not recommend convalescent plasma for treating hospitalized patients without impaired humoral immunity.
The first monoclonal antibody treatments were issued emergency use authorization (EUA) in November 2020, but there seemed to be little interest in utilizing the therapy when the focus was on the campaign to vaccinate every person aged 16 and older by federal and state governments.
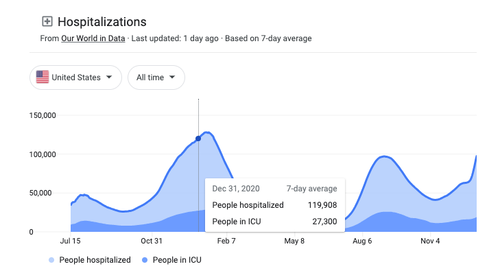
The lack of messaging around monoclonal antibody treatments from federal authorities resulted in people being unaware of the short timeframe to receive these effective therapies (within 10 days of experiencing the first symptoms) and some hospitals refusing to use them. This led to low demand for the monoclonal antibodies at a time when it should have been utilized to help alleviate strain on hospitals, as the United States was reporting over 100,000 people hospitalized for or with COVID-19 by the end of December 2020.
Dr. Peter McCullough, an internist, cardiologist, and epidemiologist, said in an interview with EpochTV’s “American Thought Leaders” program that he was “shocked with the lack of fanfare on the monoclonal antibodies, there was almost no uptake.”
He added, “We heard reports that over 80 percent of the supply was sitting on the shelves. Nursing homes weren’t informed, urgent cares weren’t supplied, hospitals weren’t in supply. There wasn’t any messaging.”
The Epoch Times reached out to the CDC for comment about the lack of messaging around the COVID-19 therapeutics and was told to contact the FDA, who did not reply by press time.
Due to the low uptake, the HHS allowed authorized health care providers to order the therapies directly from the supplier from February 2021 to Sept. 13, 2021. But the emergence of the Delta variant and waning vaccine effectiveness saw increased demand for the therapies, leading the HHS to announce it would revert back to taking control of distribution.
The number of monoclonal antibodies allocated to each state and territory would be based on the case numbers, hospitalizations, and utilizations, a distribution system that is still being used today.
In the Sept. 13 announcement, the HHS claimed that the Delta variant caused a surge in monoclonal antibody therapy uses, “particularly in areas of the country with low vaccination rates,” without further clarification.
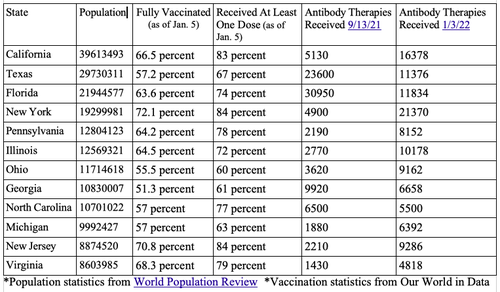
Looking at the statistics from the HHS itself and comparing the vaccination rate and monoclonal antibodies distributed to the 12 most populated states did not always show a correlation between a low vaccination rate with more uptake of the therapies.
The emergence of the Omicron variant further added to the confusion on whether certain antibody therapies should continue to be used and if they would still be effective clinically, particularly when the HHS halted the allocation of the therapies two days before Christmas.
HHS issued a notice on Dec. 23 that it would halt distribution of the REGEN-COV and Eli Lilly antibody therapies, based on in-vitro or “test-tube” studies—which have yet to be independently peer-reviewed—which found the antibody treatments may not be as effective against the Omicron variant, leaving GlakoSmithKline’s sotrovimab as the only available treatment.
The Epoch Times has reached out to both the drug manufacturers for comment. Eli Lily didn’t respond and Regeneron directed us to their Dec. 16 press release (pdf), which states, “While Regeneron’s currently authorized REGEN-COV antibodies have diminished potency against Omicron, they are active against Delta, which currently is the most prevalent variant in the U.S.”
The company said it is awaiting approval for a clinical trial of its “next generation” monoclonal antibodies that would be active against the Omicron variant and all variants of concerns.
Efficacy With Omicron?
Florida’s State Surgeon General Dr. Joseph A. Lapado says that the findings of one study conducted in a lab don’t necessarily conclude the same outcome in humans.
“So the laboratory evidence was indicating the affinity of the antibodies, such as the Regeneron antibodies for the Omicron variant were diminished … but that’s not the same thing as concluding that the Regeneron monoclonal antibodies will not work in a patient with Omicron,” Lapado said at a press conference on Jan. 3.
He added, “So, there’s a difference between laboratory data and clinical data. And they made the decision to withhold medication based on laboratory data, but we care about clinical outcomes. The decisions should be based, obviously, on clinical data, which is why they’ve reversed [the pause].”
HHS then lifted the pause eight days later on Dec. 31, allowing all states and territories to continue ordering the treatments, citing that “the prevalence of COVID-19 variants remain dynamic.” The decision was also “in light of recent National Institutes of Health (NIH) clinical guidelines published on Dec. 30, 2021, and the significant variability in the prevalence of the Omicron Variant of Concern (VOC),” it added.
The NIH is recommending a three-day course of intravenous remdesivir as a treatment option for nonhospitalized patients with mild to moderate COVID-19 who are at high risk of severe illness when Omicron is more than 80 percent prevalent in a region.
The recommendation was based on the PINETREE trial, which found an 87 percent relative reduction in the risk of hospitalizations and deaths compared to the placebo group. However, there was not a significant difference in the safety profile of both groups as “adverse events occurred in 42.3 [percent] of the patients in the remdesivir group and in 46.3 [percent] of those in the placebo group.”
Remdesivir is approved by the FDA for patients requiring hospitalization. Experts recommend physicians perform “close kidney monitoring when prescribing remdesivir” and be aware that the drug may cause kidney disorders. In addition, the NIH says that every patient should be given a liver function test and a prothrombin time test (to measure the length of time it takes blood to clot) prior to receiving remdesivir.
Tyler Durden
Fri, 01/07/2022 – 18:40
via ZeroHedge News https://ift.tt/3HKoZRf Tyler Durden