Pfizer Begins Human Trials For New Omicron Vaccine As US Cases Tumble
Pfizer and its partner BioNTech just launched the first human trials of their revised COVID vaccine which has been adapted to try and provide better protection against the omicron variant. Shortly after the emergence of omicron in late November, Pfizer and its rivals all promised to produce another generation of vaccines, claiming they could have them ready within three months.
So far at least, that timeline appears mostly on track. However, an even more important question for Pfizer might be this: who will even need these jabs when they’re ready, since it looks like the omicron wave numbers will have finally tapered off by the time the trials are completed.
At any rate, the two firms said Tuesday they had begun enrolling adults ages 18 to 55 for trials taking place in the US and South Africa. The trials will seek to examine the safety, tolerability and, most importantly, the immune response generated by the new vaccines, per WSJ. The big question will be whether they do what their makers have created them to do, or not.
At least one human subject has already received a shot of the new vaccine, Pfizer said Tuesday. Initial trial results are expected some time during the “first half of the year”. But Pfizer CEO Albert Bourla has said that the company could receive approval for the jab from federal regulators as early as March.
Dr. Fauci still believes it’s “entirely conceivable” that a fourth booster will be needed, as we reported the other day. And speaking on MSNBC earlier, the good doctor warned that it would be “prudent” to prepare for the likelihood that omicron will persist at pandemic levels for some time. He even stressed that an omicron-focused vaccine would help humanity prepare for the eventuality that the omicron-driven wave persists.
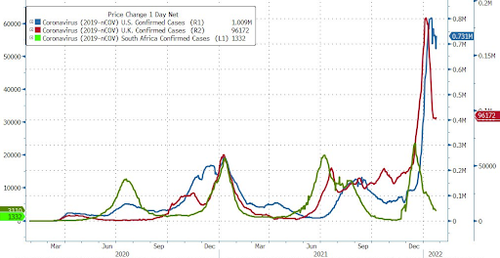
However, case numbers have fallen substantially in South Africa, while also appearing to have turned the corner in the US, UK and elsewhere across Europe, globally, the number of newly confirmed cases of the virus has continued to rise.
Despite Bourla’s cheery rhetoric about the possibility of only requiring one COVID booster per year, US regulators are already questioning whether more jabs are even necessary it seems. US health authorities have said that boosters targeting omicron might not be needed, since it’s unclear whether future dominant variants will be descents of omicron, or other strains.
Research shows that certain mutations in the omicron variant allow it to more effectively bypass what little protections vaccines from Pfizer and Moderna actually offer.
As for the new trials, the two firms said they would test how the vaccine performs in three groups of volunteers, with some of the subjects coming from the study that led to the current vaccine’s clearance in 2020. Researchers plan to enroll up to 615 people who received the two-dose primary series of the current vaccine 3-6 months prior to their enrollment.
Once enrolled, they will receive either one dose of the omicron-focused vaccine, two doses four weeks apart from each other, or a third dose. Additionally, researchers will also recruit up to 600 people who received three doses of the current vaccine 3-6 months ago. These subjects will receive either one dose of the omicron jab, or a fourth dose of the current shot.
Tyler Durden
Tue, 01/25/2022 – 17:35
via ZeroHedge News https://ift.tt/3u0uLdF Tyler Durden