Despite FDA Claims Of Clamping Down On COVID-19-Scams, One Dodgy Company Remains On Its Website
Early on during the coronavirus pandemic, the FDA had been criticized for its restrictive approach for testing. As a result, it wound up swinging the other way, allowing virtually any manufacturer to develop and distribute serological (antibody) tests.
The agency established that manufacturers simply needed to notify the agency that it was offering tests and include basic boilerplate disclaimers such as the products not being FDA approved.
Naturally this led to a flood of fraudulent tests and shoddy products being made available throughout the U.S. and imported from China. There are now about 160 serological tests being marketed across the country that have not received formal authorization from the FDA.
And so, the FDA has now said it is clamping down on test manufacturers making exaggerated claims and will require manufacturers to submit data validating the accuracy of its tests. But some dodgy actors remain on the FDA’s website, including what appears to Zero Hedge to be some low hanging fruit.
Take, for instance, Promedical Equipment Pty Ltd, a relatively unknown company prior to the coronavirus pandemic. Prior to the coronavirus, the company specialized in “cryogenic, massage therapy and erectile dysfunction machines.”
Promedical has been the subject of a series of investigative articles by The Guardian (like this one and this one), who pointed out in late April that the company – headed up by a convicted rapist with “no obvious experience in medical diagnostics” – was under investigation by Australian medical regulators.
The company had reportedly entered into a contract with the Australian government to sell them 500,000 coronavirus tests made by well-known Chinese manufacturer, Guangzhou Wondfo Biotech. The Chinese test had already been approved for use in Australia and the U.S. and Promedical was acting as the middle-man.
But then, the relationship between Wondfo and Promedical broke down in spectacular fashion, with Wondfo revoking authorization to distribute its products from Promedical. The order for the Australian government was never fulfilled.
“To date, the tests have not arrived in Australia and no payment has been made,” a government spokeswoman said in April.

Puerto Rico, which is in the midst of a “testing crisis”, saw a similar deal with Promedical collapse.
The company had promised the Puerto Rican government a large quantity of tests as part of a $38 million deal. The company promised Puerto Rico “large shipments” but at the time only held “a small batch” of testing kits in its Brisbane warehouse, according to the Guardian.
In the interim, Bradley Mayo, former manager of exports and logistics for Promedical, said he has distanced himself from the company. He told the Guardian:
“In these unprecedented and extremely challenging times, the medical supply chain industry is volatile at best, with many opportunists who aim to use this time of crisis to … enrich themselves. After lengthy investigation and due diligence, I have decided to distance myself from Promedical.”
In addition to being a convicted rapist, Promedical CEO Neran de Silva has reportedly also made claims to be a doctor, posting qualifications from the University of Queensland and Griffith University on his LinkedIn. The Guardian says that neither university has a record of him in their online graduation verification systems.
de Silva’s previous venture, a cryo company, also collapsed.
Finally, on Thursday morning, The Guardian reported that Promedical had been fined $63,000 for “false claims” about their test kits.
But the U.S. FDA, which claims to have an eye out specifically for these types of potentially dodgy companies, hasn’t seemed to take notice.
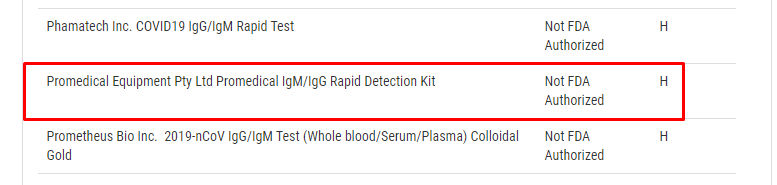
While reviewing the list of test manufacturers on the FDA site listed under the question “Q: What commercial manufacturers are distributing serology test kits under the policy outlined in Section IV.D of the Policy for Coronavirus Disease-2019 Tests? (Updated 5/6)”, Promedical’s name pops right up:
“Promedical Equipment Pty Ltd Promedical IgM/IgG Rapid Detection Kit”
Tyler Durden
Fri, 05/08/2020 – 17:05
via ZeroHedge News https://ift.tt/2SL92mU Tyler Durden