Hydroxychloroquine Demand Triples Overnight Following FDA Approval
The Food and Drug Administration gave emergency-use authorization to hydroxychloroquine as a treatment for the coronavirus pandemic on Sunday. But demand for the drug backed by President Donald Trump that is typically used to treat malaria soared prior to the move, according to data from Symphony Health.
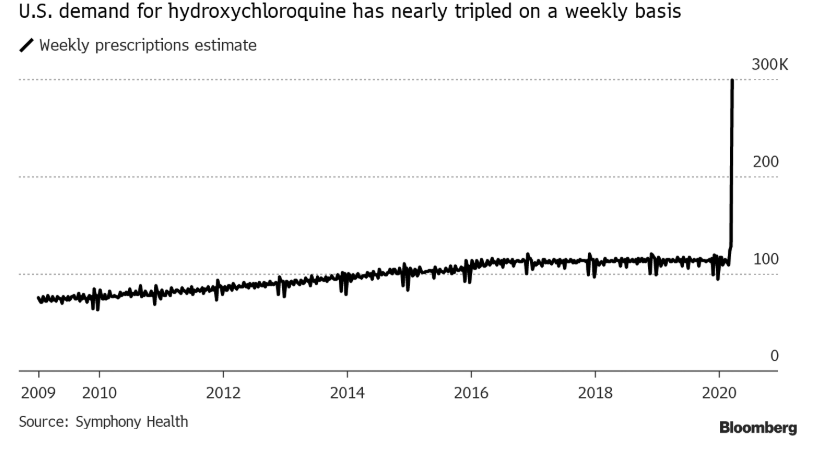
Weekly prescriptions soared from 100k to 300k over the past week, since President Trump first mentioned the drug as one of two commons drugs that produced potentially wondrous results if administered together (the other drug, azythromycin, better known as a Z-pak) to COVID-19 patients.
Given the market’s desperation for anything that might cure a disease that is killing 1 in 10 people it infects in certain areas, it would seem that concerns of those who took these medications regularly before the crisis might be struggling to source their medication for the first time.
The FDA gave emergency approval to a Trump administration plan to distribute millions of doses of anti-malarial drugs to hospitals across the country on Monday, saying it is worth the risk of trying unproven treatments to try and slow the progression of the disease.
There have been only a handful of anecdotal studies detailing a possible benefit of the drugs, hydroxychloroquine and chloroquine, to relieve the acute respiratory symptoms of COVID-19 and clear the virus from infected patients. If these effects could be widely replicated, it would be nothing short of miraculous. And the evidence certainly offers reason to hope, at least for some.
Interestingly, prescriptions for the drug surged during the week ended March 20, asnearly 300,000 prescriptions were written in the US that week, roughly triple the 113,000 weekly average in 2019.
We suspect these numbers will only continue to climb.
Tyler Durden
Mon, 03/30/2020 – 21:25
via ZeroHedge News https://ift.tt/3dGEPOI Tyler Durden