Moderna Publishes COVID-19 Vaccine Trial “Blueprint” To Quiet Concerns About Rushed Approval
Tyler Durden
Thu, 09/17/2020 – 13:05
With the AstraZeneca-Oxford vaccine study still paused in the US, reports about a sickened patient in the UK have undermined credibility with the public at an extremely delicate time. Vaccine maker are trying to achieve emergency regulatory approval, then produce hundreds of millions of doses, all by mid-next year, at the latest.
In reality, nobody can say for certain how long it will take for a vaccine to be widely. But in the meantime, companies like Moderna, which has seen its share price explode this year after taking nearly $1 billion in US taxpayer money (though that hasn’t stopped it from considering a price per dose at the upper end of the range), are doing everything they can to build back that trust.
So Dr. Tal Zaks, Moderna’s chief medical officer, consulted an outside expert, who recommended that the company be more “transparent to the point of discomfort.”
In keeping with that mantra, the company has released a 130+ page status report on its Stage 3 trials.
“If what you want to do is see the protocol – here,” Dr. Zaks said.
Moderna hopes competitors like Pfizer, which is developing a vaccine candidate in a joint project with Germany’s BioNTech, will adopt a similarly “transparent” approach. The company announced earlier this week that it’s trials have been running swimmingly.
The release of the company’s vaccine trial protocols coincided with a call to investors on Thursday.
Not all the feedback was positive. One independent research quoted by the NYT said Moderna risks exaggerating the vaccine’s effectiveness with how it processes the data.
Dr. Eric Topol, a clinical trial expert at Scripps Research in San Diego, gave the company “big kudos” for sharing the information, but said that he was disappointed by some of the details. For example, the company intends to include in its data people who developed relatively mild cases of Covid-19. Dr. Topol said more compelling evidence of the vaccine’s effectiveness would be produced if the company counted only moderate to severe cases.
In addition, the protocol allows for the possibility of stopping the trial early after a relatively small number of cases. Stopping early could lead to an exaggerated perception of the vaccine’s efficacy, and could also miss safety problems that could turn out to be significant later if the vaccine is given to millions and millions of people.
“Take the time, the extra weeks,” Dr. Topol said. “No shortcuts. Nobody will regret it. I’ve been doing clinical trials for decades. I don’t know if there’s ever been a more important one than this one. I’d like to see it done right, and not stopped early.”
Dr. Zaks added that he’s “proud” of what’s going on: “I’m proud of doing that…I don’t think there’s much there that we’re disclosing that hasn’t already been spoken to, but let the public be the judge of that.”
Below, we have summaries of the most important takeaways from the protocols, culled from a NYT report on the release (text courtesy of the NYT).
- The company’s coronavirus vaccine, developed in collaboration with scientists from the National Institutes of Health, was the first to be tested in humans. The Phase 3 study now underway has enrolled more than 25,000 of its intended 30,000 volunteers, and Dr. Zaks said the enrollment should be complete in the next few weeks.
- About 28 percent of the participants are Black, Latino or from other populations that have been particularly hard hit by the disease. A diverse enrollment has been considered essential to make sure that the findings apply to people from as many backgrounds as possible.
- Half the participants receive the vaccine, and half a placebo shot consisting of salt water, with neither the volunteers nor the doctors treating them knowing who gets which. Two shots are needed, four weeks apart. Then the participants are monitored to see if they develop symptoms of Covid-19 and test positive for the virus.
- Side effects of the vaccine are also tracked, with participants recording symptoms in electronic diaries, taking their own temperatures, making clinic visits and receiving periodic phone calls to assess their condition. In earlier studies the vaccine has caused transient reactions like a sore arm, fever, chills, muscle and joint pain, fatigue and headaches.
- To determine the vaccine’s efficacy, Covid-19 cases are counted only if they occur two weeks after the second shot. Some patients are already two weeks beyond the second shot, but Dr. Zaks said he did not know if any trial participants had contracted the disease yet.
- A total of 151 cases — spread between the vaccine and placebo groups — would be enough to determine whether the vaccine is 60 percent effective. The Food and Drug Administration has set the bar at 50 percent.
(Source: NYT)
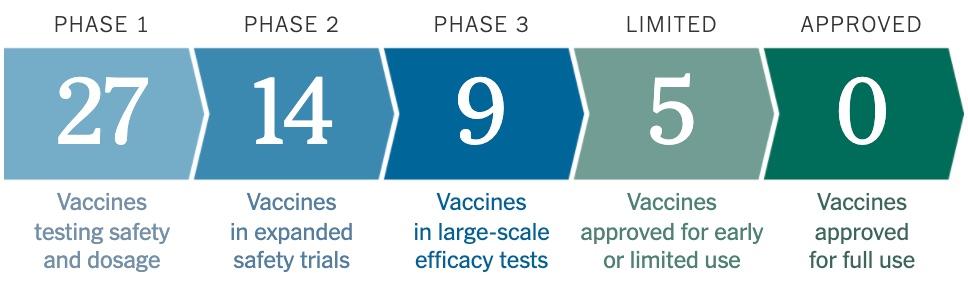
According to the NYT’s vaccine tracker, 9 companies, including Moderna, have sheparded their vaccine efforts into Phase 3 – the final phase.
Source: NYT
Moderna is developing a vaccine based on a new technique that uses messenger RNA to produce viral proteins in the body that would help it fight off the virus.
Why do vaccine-makers feel the need to justify these projects like this? Well, if you don’t know, try asking Bill Gates.
via ZeroHedge News https://ift.tt/32GvWB7 Tyler Durden