Last month, Seattle’s city council voted to lift a host of restrictions on many home businesses, thanks to the efforts of one small local cidery and its supporters.
As I detailed in a February column, after one neighbor complained repeatedly to the city about Yonder Bar, the city forced the “bar”—a converted home garage walkup window where consumers may only purchase cider to drink at home, rather than a place where people drink alcohol beverages—to close temporarily.
As I also explained in the column, the city, which had approved and licensed Yonder Bar prior to its opening, claimed, in closing down the bar, that Yonder Bar was operating illegally in a residential area, didn’t have adequate off-street parking, used signage to indicate to consumers that it’s a business—and not, say, an unmarked garage full of old tires or broken croquet mallets—and could operate only by appointment.
That might have been the end of the story. But thousands of Yonder Bar’s neighbors and at least a couple city councilors rallied behind the takeaway-only bar. Those city councilors introduced a bill that would not just allow Yonder to reopen—but also allow other, similar home-based businesses to operate without fear of being shuttered by the city.
City Council members Dan Strauss and Lorena Gonzalez introduced the bill, dubbed Bringing Business Home, in order to “provide additional support and a means towards economic recovery for small businesses adversely affected by current land use codes during the pandemic.”
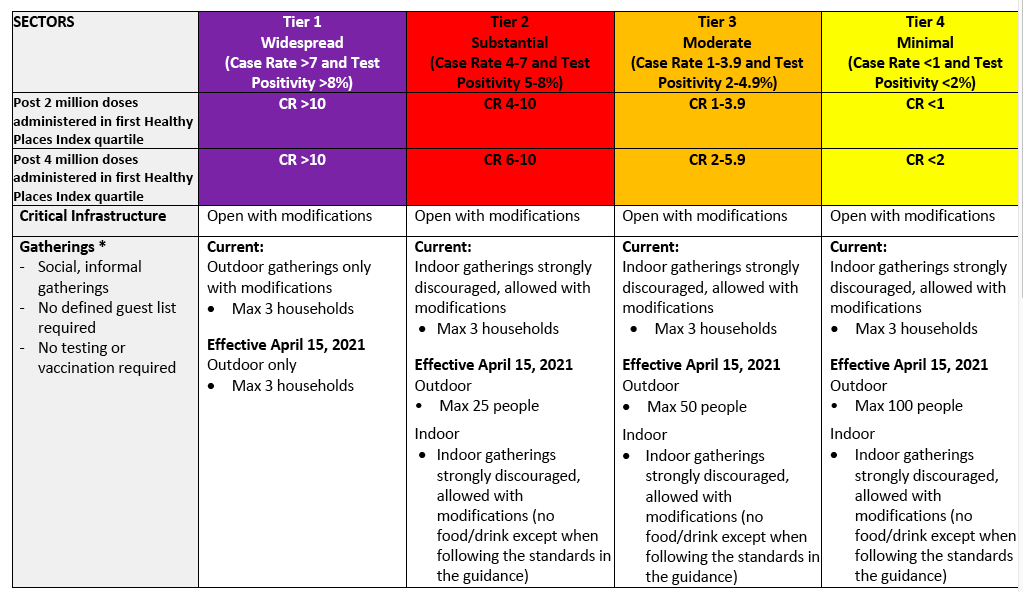
The bill was intended to lift some of the most onerous home-business requirements, including the appointment-only rule, signage ban, cap on employees, and traffic restrictions.
In my column on Yonder Bar, I cited one local Twitter account that predicted Yonder Bar’s persistent nemesis could end “up getting a citywide zoning change that will legalize stuff like Yonder on every lot.”
That’s largely what happened. Yonder Bar reopened soon after the bill was introduced. And then, rather amazingly, the Bringing Business Home bill passed by an 8-1 vote.
“This bill removes one of the biggest hurdles for small businesses—commercial rent—and gives them the opportunity to follow their dreams,” Yonder Bar owner Caitlin Bramm told me this week. “We started Yonder Bar in our garage, and it allowed us to safely and confidently grow while providing valuable cash flow for our business. We are thrilled to have it back open, and can’t wait to see what opportunities this bill opens up for others.”
“Our land use code cannot be the barrier to vibrant neighborhoods and a strong economy,” Councilmember Strauss said after the bill’s passage. “It’s essential we meet our businesses where they’re at: whether that’s out of their homes or garages.”
Strauss is right. But given that the legislation is only temporary—it’s set to expire in a year—the city council still has work to do to ensure Seattle’s government isn’t a barrier to the success of small food entrepreneurs.
While many small food producers across Washington State face similar restrictions, there’s also a movement currently underway to expand opportunities for home food entrepreneurs throughout the state. A bill to legalize microenterprise home kitchens, which passed in the legislature last month and is now before a key state Senate committee, could turn some home-based cooks “into a legitimate industry, fostering entrepreneurs and lowering the barrier to entry into the food industry.”
The bill’s sponsor, State Rep. Noel Frame, who also hails from Seattle, explained last month that small entrepreneurs “really want to get into the food business but may face pretty big barriers to do so, particularly cost. This is a pathway of opportunity for them that is slightly lower-barrier.”
Frame’s bill could help bring in from the cold underground food sellers such a “C.,” an anonymous seller I contacted (while researching a 2018 column) through Facebook’s Marketplace. I subsequently met C. in a Costco parking lot to buy a few of the delectable tamales her mom cooked to help supplement her income.
Unfortunately, even if Frame’s bill passes, it’s more likely to leave C. and her mom and thousands of other existing and potential home-food entrepreneurs out in the cold than it is to legalize their work.
That’s because, as part of a pilot program detailed in the bill, Washington State’s largest county—King County, which includes Seattle—may issue no more than 30 permits during the law’s first year. Most other counties could offer only 10 such permits. Other restrictions include a cap on the number of meals a seller may offer each day or week. Also, the bill wouldn’t take effect until summer 2022.
These limits have unfortunately become a feature of microenterprise home kitchen laws. California, the first state to adopt such a law, has seen its new policy vacillate between flailing and failing, largely because the state left it up to local governments to opt in to the law.
“While a handful of counties and cities have expressed interest in adopting the law in their own jurisdictions, no California city or county save Riverside County—not one—has adopted the law and drafted rules to implement it,” I explained in a column last year on the California law’s status, dubbing it “nothing more than a cruel illusion.”
Seattle city council members deserve credit for recognizing that laws and regulations often act, as Councilor Strauss noted, as a “barrier to vibrant neighborhoods and a strong economy.” Washington State lawmakers deserve credit, too, for recognizing that home-based culinary entrepreneurs want and deserve a path to legitimacy.
But until and unless Seattle’s home-business law is made permanent, and Washington State’s microenterprise home kitchen bill is strengthened, streamlined, and adopted, Washington State food entrepreneurs will likely remain tangled in red tape.
from Latest – Reason.com https://ift.tt/3sP0aN1
via IFTTT